Behavior is the functional
component of evolutionary change. How
well an animal runs is the selective force, not its legs. Paleontologists study the evolution of hard
parts because those are what fossilize.
Studying changes in femur lengths, however, leads to the misconception
that it is legs that evolved, rather than running or jumping. For biologists, the evolution of dog
behavior is found in the mechanisms of evolutionary change from the antecedent
wolf behavior.
Studying the evolution of dog behavior implies some assumptions. First, one assumes that something called dog
behavior can be delineated and that it varies somewhat among dogs. People often discuss dog behavior or dog
training as if it were the same for all dogs.
Second, the origin of some of these variations is assumed to be
genetic. What evolves is the genotype,
the specific arrangements of genes. The
only material that can be passed from generation to generation is genes.
BREED-TYPICAL
BEHAVIORS
Professional dog breeders and trainers believe that inherent
differences in behavior exist among dog breeds. Breeders select animals that display the breed-typical behaviors,
whereas trainers direct the expression of those innate behaviors. For example, trainers do not train border
collies to eye sheep, or setters to point birds, or retrievers to
retrieve. These behaviors emerge
spontaneously during ontogeny (i.e., the course of the life of an individual). Training commences after the emergence of
the innate behavior; the trainer teaches the dog when and where to display the
behavior.
Breeds are often classified by the job they were selected to perform,
such as hunting, herding, or guarding.
They are further subclassified as coon-hounds, pointers, or upland
retrievers; heelers, headers, or huntaways; and people-guardians or
flock-guardians. It is assumed that
each breed has a unique set of behaviors that endow the members such that no
other animal could be taught to perform the task as well. Not everybody agrees. Black and Green hypothesized that social
attachment and reduced interspecific aggression between dogs and sheep could be
achieved using dogs of any lineage if they were raised properly. These conclusions were based on observations
of Navajo dogs that were used to guard sheep but supposedly lacked long-term
selection preparing them to perform this task.
UNNATURAL
SELECTION
Selection in dog breeding differs from Darwinian or natural
selection. Darwinian change results
from differential survival and reproductive success among individuals in a
geographically isolated population; In contrast, dog breeds are created by
humans selecting dogs of a particular phenotype (i.e., with a particular set of
observable characteristics and that perform a particular task) and separating
them from the rest of the canine population for breeding purposes. Breeders identify a few single-gene
characteristics, such as coat color or achondroplasia (short limbs), as a breed
marker, which all animals of the breed must have– regardless of the
adaptiveness of the characteristic.
Breeds are hybrid saltations, often perfected in a few
generations. Darwinian selection takes
longer, perhaps millions of years.
Crossbreeding creates not averages but new phenotypes that can be maintained
as new breeds.
DIFFERENT
BREEDS– SAME GENES
Dogs of all breeds have the same karyotype (number and shape of
chromosomes) and produce reproductively viable hybrid offspring. The differences among breeds are not gene
differences (i.e., different arrangement, ordering, or number of
genes). Phenotypic (including
behavior) differences among dog breeds are produced by slight allelic
differences in gene products in three categories: ontogenetic onset (timing of the activation of the gene),
quantity of gene product, and ontogenetic offset (timing of deactivation of
the gene).
No gene arrangement has yet been identified that would permit
identification of any breed or any particular behavior. To our knowledge, attempts to detect
"fingerprint" genes for pit bull terriers, or for aggressive
behaviors in pit bull terriers, have so far failed. Mitochondrial DNA investigations give no clue to relatedness
between breeds, nor do they group breeds in any of the well-known categories,
such as sporting, working, or terrier.
Rather, they reveal what has always been obvious but is usually
ignored: dog breeds are local and
temporal phenomena recently derived by crossing other breeds or local
variants. Any handbook of dog breeds tells
what breeds were used to create a new breed at the turn of the twentieth
century or what breeds were used to improve an old breed.
In addition to chromosomal and mitochondrial similarities, most members
of the genus Canis have conservative phenotypic similarities (e.g.,
palate to skull length2) and also fundamental similarities in social
and reproductive behaviors.35
This information suggests that despite the claim that Canis
familiaris is one of the most varied species on earth, the evolutionary
distance between dog breeds or within the genus is short– perhaps even
nonexistent.
DIFFERENT
BREEDS– DIFFERENT TEMPERAMENTS
Differences in behavior among dog breeds are often given as differences
in temperament. The combined results
of these studies indicate some variation in emotionality, vocalization,
activity, problem-solving, reaction to human handling, and trainability. Mahut, Borchelt,2 and Hart and
Hart13 reported breed differences in temperament among pet dogs
raised in private homes. They assumed
that these differences were largely innate because pet dogs of all breeds are
raised similarly.
MOTOR
PATTERN AND BREED BEHAVIOR
Motor pattern differences among dog breeds have also been reported.14 Studies of motor patterns concentrate on
the kind of behavior, its frequency, and the sequence in which the motor
patterns appear.
The kind or quality of the behavior refers to the action the animal
performs in reaction to a specific internal or external signal. Ideally, this would be done by sequencing
anatomical events (e.g., muscle movement); but usually, the composite behavior
is simply described: "eye" in
herding dogs, "point" in pointers, heeling in heelers. Male dogs raise a hindlimb during urine
marking. Because their relatives, male
wolves, also display this behavior but members of other similarly shaped
species (e.g., bears) do not, the behavior is assumed to be not only an
inherited motor pattern but a taxonomic characteristic.
Two species, or two breeds, may differ not in the kind of motor
patterns but in their frequency of expression. Wolves rarely bark, whereas barking is ubiquitous among
dogs. Nevertheless, greyhounds in Argentinean
villages rarely bark at strangers, whereas maremmas in Italian villages rarely
refrain from barking at strangers.
Behaviors also change frequency ontogenetically. Wolves bark more frequently as young
adolescents and rarely as adults. Dog
pups bark first at 7 days of age and frequently thereafter throughout ontogeny.
The behavior sequence in which motor patterns appear differs between
species and between breeds. Dogs that
work with sheep provide an excellent example.4 Among border collies (a sheep-herding breed)
a predatory eye-stalk motor pattern follows a

Figure
1: Border collie showing
"eye" to sheep. This behavior,
with its characteristic "stalk," causes sheep to bunch and move away
from the dog.
predatory
orientation 85% of the time, whereas an investigative or a social sequence
commonly followed such an orientation among livestock-guarding dogs. Border collies displayed eye-stalk-chase
behaviors both inter- and intraspecifically.
Some authors believe that the eye-stalk-chase motor patterns are homologous
to those of wolves. Most European
sheep-guarding breeds (e.g., Great Pyrenees, maremma, and Anatolian shepherd)
do not display these predatory sequences (Figure 1).
CRITICAL
PERIODS OF BEHAVIOR
Environmental factors cause behavioral variation among dogs. Scott5 and Fox6 found
that puppies undergo a critical period of socialization when they are
approximately between 3 and 16 weeks of age.
According to these authors, dogs are susceptible to permanent
alterations of behavior as a result of environmental stimuli during this stage
of development. Cairns and Johnson18
and Fox5 found that social conditioning with another species
often results in unusually low levels of aggression toward the other species
and development of a social attachment, even between species that might
normally have a predator-prey relationship (e.g., dogs and sheep).
The critical period hypothesis is evidence for genetic predispositions
for development of specific behavioral repertoires (see the article by
Estep). For example, we raised
livestock-guarding dogs with sheep and minimized pup-human contact during the
critical period when social motor patterns emerge. Some of these pups became good flock guardians but were
permanently shy around people. These
dogs were difficult to manage, but they were less likely to harass strangers
in or near the pasture. How much
handling by humans dogs need in order not to become people-shy is also a
genetic variable. We have had strains
of dogs predisposed to this shyness; and within those strains, siblings raised
in the same pen can be shy or not shy, with the same amount of human contact.
BIOLOGIC
DETERMINANTS OF BEHAVIOR
To understand any behavior, whether motor patterns, critical periods,
or temperament differences, one must discern the underlying biologic determinants. Differences in innate behavior between
breeds can ultimately be traced to biologic differences. These may be gross differences in size or
shape or minute differences in the chemical structure of a neurotransmitter or
a hormone.
A breed is defined by a structural standard (e.g., a Siberian husky
should not weigh more than 60 lbs [27 kg]).
This standard was derived through a process of selecting breeding stock
from among dogs that performed the task of pulling sleds. Thus, innate behavior implies the
structural capacity to perform.
Behavior is shape and size moving through space and time. This definition may seem simplistic. The evolution of breeds, however, is the
selection among allelic variations of genes; but genes simply produce enzymatic
reactions for the production of proteins, which give the organism shape and size. The genetic basis of behavior is a
gene-encoded shape that allows the animal to perform in a particular way. The shape allows, but also limits, the
performance. In the following example,
sled dogs provide a simple illustration of how size (and consequently shape)
determines a particular kind of performance.
Racing sled dogs are the world's fastest quadrupeds for distances over
17 km. Dogs on championship teams weigh
less than 25 kg. Sled dog drivers would
like to use bigger dogs, because big dogs have longer gaits (more reach) and
cover more ground with each stride, but bigger dogs do not seem to do
well. Experiments by Phillips and
coworkers9 showed that bigger dogs suffered greater heat stresses
because surface-to-volume ratio is geometric and not linear with size
change. Big dogs have more heat-storing
capacity because they have a larger volume compared to radiative surface
area, which prevents the dissipation of heat during fast long-distance running.
Greyhounds (30 kg), which are among the fastest of dogs, are limited to
sprints. Their racetracks are either
3/8 or 5/16 of a mile. Like human
sprinters, they finish their races in a matter of seconds (31 seconds is a
common finishing time); so heat load does not limit performance.
Sled dogs have walking gaits (pace, trot, or lope), in which at least one paw is always on the ground.
Greyhounds have running gaits, a series of leaps into the air
with all four feet off the ground.
Running (leaping) is a fault in sled dogs. Dogs that run (called floaters) are at a severe
disadvantage because the back-strap pulls them off balance when all four feet
are off the ground (Figure 2).
Racing sled dogs have gait and size characteristics that allow them to
run long distances efficiently. These
characteristics are innate because they are genetically (allelically)
determined. The shape of their
shoulders and pelvic girdles and the length of their back and their size are
the products of their genes. All those
differences in shape between the greyhound and the sled dog allow each of them
to perform exceptionally well in their own environment but limit their
performance in the other breed's environment.
One cannot teach the wrong breed to excel at the wrong task. A greyhound cannot pull a sled at racing
speeds for long distances. Not only
would the greyhound overheat, but it would be awkward and inefficient in
harness because of its gait. It would
be difficult (and probably uncomfortable for or even harmful to the dog) to
train a greyhound to run with at least one foot on the

ground.
Similarly, no amount of training or conditioning would enable a basset
hound to achieve the speeds of a greyhound.
Structural variation is the biologic basis of behavioral variation.
LIMITS
OF STRUCTURAL VARIATION
Breeds
differ not only in gross conformation, size, muscle, bone, and hair, but also
neurologically and hormonally. Arons
and Shoemaker10 searched for the structural differences (quantity
and anatomical distribution of neurotransmitters) underlying differences in
motor patterns among breeds. They found
differences in motor pattern and motor sequence among sheep-herding dogs,
sheep-guarding dogs, and Siberian huskies.
They then attempted, with some success, to correlate these breed
differences with neurotransmitter patterns in the brain.
Their data suggest that breed-typical motor patterns reflect
differences in the distribution and quantity of neurochemicals. Their findings not only support the
assumption of breed-typical anatomical differences but are parsimonious with
the direction of those differences. Low
levels of dopamine correlated with the more lethargic temperament of
livestock-guarding dogs. This contrasted
with higher dopamine levels in the hyperactive border collies and huskies. Additionally, a lack of this
neurotransmitter in a particular region of the brain resulted in a lack of
excitability in organs stimulated by that portion of the brain. The animal therefore cannot be expected to
increase the rate of motor function through training or conditioning. Anatomy, whether structural or chemical, not
only allows breeds to perform in a particular way but also limits behavior.
ONTOGENY
OF BEHAVIOR
If a dog's anatomy changes over its lifetime, then the “innate”
behavior must also change. Dog behavior,
then, must also be defined ontogenetically.
As a dog changes anatomically (its size, shape, hormones, and neurons)
from neonate to puppy to juvenile to adult, it goes through stages of
behavioral changes. The synonyms grow
up and develop misleadingly imply that ontogenetically a dog goes
from a primitive to a more advanced state.
In fact, neonates are behaviorally complex– just as or even more complex
than adults. Many people think of
ontogenetic changes as growth or maturation, but the changes might better be
thought of as metamorphosis from one complex stage to another.
Each new structural organization of the animal produces a new set of
innate behaviors. Thus, one must
specify the dog's current ontogenetic stage when discussing its behaviors. Too often, people think of dog behavior as
adult dog behavior, assuming that the puppy "develops" into the
adult, that puppies are less than perfect, and that the purpose of life is to
mature into an adult.
TWO
COMPLEX INNATE BEHAVIORS
Alarm calls by neonates and puppy retrieval behaviors by dams are
innate, reflexive, hard-wired, complex dog behaviors that are ontogenetically
limited (i.e., limited to a particular time in the life cycle). Neonates of all breeds can give species-specific
alarm calls from the moment they are born.
One is a care-soliciting call, signaling "I am lost." This
call is an innate behavior, a specific motor pattern. It is stereotyped and can be sonographically measured and
described. It has a single “meaning”
and is only given in one environmental context. Its onset is from the moment of birth. There is no learning period.
We observed a puppy still attached to its placenta giving this call,
having been abandoned by its mother in a pasture. She had departed to the barn to have the rest of the
litter. Puppies continue to emit the
call until they are retrieved or until they weaken and perhaps die. The call is unchanging from the first to
the last vocalization.
Offset (cessation) of the "lost call" occurs before 5 weeks
of age. Neither practice nor reward
prolongs the behavior into adolescence or adulthood.
Puppy retrieval by mother dogs is similarly hard-wired. In our experiments, the onset of puppy retrieval
was after the last puppy was born. Onset
varies in other species. Experiments
with rats showed that they can exhibit pup retrieval several days before parturition. This motor pattern, displayed only by a dam
(in dogs), results from the specific neonatal vocalization given by a
pup. One of our border collies retrieved
and returned to the nest a small tape recorder producing this
vocalization. Other environmental
signals also initiate the response, for dams sometimes retrieve dead puppies.
Offset of puppy retrieval averages 13 days after parturition. The dam no longer responds to these vocalizations
from puppies after that day, even though puppies may emit the signal in
contextually appropriate circumstances for several more weeks.
In both pup and dam, the motor patterns are intrinsic. There is no learning or development
period. The behavior is
"perfect" the first time it is displayed, the emergence is
spontaneous, and it is contextually and motivationally relevant and
specific. Neither behavior occurs in
other behavioral contexts, such as play or courtship. Neither other puppies nor males respond to the retrieval
call. Nor do adults, of either sex,
emit them.
The fact that the mother dog retrieves a tape recorder during the
critical period but not a calling pup after the critical period suggests that
no cognitive process is occurring. The
call is a reflex in neonates, and the retrieval of the calling pup is a reflex
by the dam. Both motor patterns have a
critical period of display. They are
not elicited before or after the particular ontogenetic stage, although the retrieval
behavior can be induced hormonally in experiments.
PHYLOGENY
AND HOMOLOGIES
The care-soliciting calls of pups are not just species-specific but
identical across the genus and similar across family and order. In other words, wolf cubs and dog puppies
have the same set of care-soliciting calls, and mammals in general have similar
care-soliciting calls. The similarity
between them suggests that the ancestors of dogs and wolves had this same set
of modulating tonal signals.
The care-soliciting calls of puppies and cubs are phylogenetically
related and are said to be homologous.
The genes that build the structures that allow this reflexive behavior
to be displayed were inherited by dogs from their wolf ancestor and by wolves
from their carnivore ancestor. That
particular pattern of genes has probably been around for millions of
years. Since the call induces the
retrieval response, the two behaviors probably coevolved. Surely they are hard-wired; if searched,
canine anatomy would reveal neurologic and hormonal arrangements that
predispose dogs to display these behaviors in the presence of an appropriate
environmental stimulus.
Because these behaviors coevolved, one is not more primitive nor is the
other more complex. Mammary glands
evolved, and so did specialized neonatal mouths that suck on them. Care-soliciting behavior evolved with
care-giving (maternal) behavior. The behavior
of the neonate is just as old and just as complex as the coevolved behaviors
of the adult. The main point is that
the emission of the retrieval signal by the puppy is equally as complex, in
evolutionary terms, as the retrieval response of the mother. In fact, mammalian neonatal behavior is a
recent evolutionary advance in vertebrate evolution. The pup is not a primitive form that "develops" into a
behaviorally advanced adult.
For this discussion, just one behavior each was picked from among many
in the neonatal and adult repertoires.
During parturition, females remove puppies from the placenta; and they
do it perfectly (with rare exceptions) with their first pup. Pups released from the placenta find a teat
and begin to nurse. Sucking is a complex
motor pattern that adult dogs cannot perform.
HOMOLOGOUS
BEHAVIORS AND MOTIVES
When describing a dog's behavior, an ethologist uses motor patterns to
try to determine the animal's motivation.
To say that a dog killed a sheep or bit someone does not help in
understanding why the animal did it.
Unless one knows why the dog killed the sheep, one cannot begin to
correct the problem.
Dogs have many different innate bites; that is, they have several motor
patterns that are described as bites.
To understand what may have motivated a dog to bite, investigators need
information on the quality of a bite:
where it was directed and what other motor patterns were displayed in
the sequence.
Canid behavioral repertoires include several predatory biting motor
patterns. Wolves string these patterns
together in feeding or foraging behavior.
A typical stereotyped predatory sequence in a canid is: Orientation toward prey → eye →
stalk → chase → grab bite → crush bite (kill) →
dissecting bite → consuming bite.
A field biologist can often tell which predatory species killed an
animal by observing where the grab bite, kill bite, or dissecting bite appears
on the carcass. Typically, wolves and
dogs direct the crush bite at a femoral artery– the victim then bleeds to
death. Coyotes more frequently direct
the kill bite toward the carotid arteries.
Pumas suffocate their prey.
The dog family has other characteristic bite motor patterns besides
those used in the predatory routine.
Intraspecific (within species) dominance bites are directed to the top
of the neck of an animal refusing to submit, to the loin in a fight, or to the
abdomen of a submitting animal. When a
farmer tells us that the dog he hoped would guard his sheep is biting or even
killing them instead, we first try to determine the dog's motivation by noting
the motor patterns. If the sheep are
torn on top of their necks or their abdomens, we suspect that the dog is using
intraspecific dominance bites interspecifically. Confidence is bolstered if further investigation reveals other
playful motor patterns (e.g., either play bow or the dog's head and tail held
up).
Directing these behaviors toward sheep may seem inappropriate until one
realizes that the dog spent its critical period of socialization with sheep and
"learned" to display normal intraspecific behaviors toward sheep. As dogs mature sexually, they sometimes
show reproductive dominance routines toward sheep, including sexual
mountings. Because these are not the
motor patterns of a predatory routine, one can assume that the dog is not
trying to kill the sheep but has successfully bonded with them and might still
be trained to be a good sheep guardian.
If the bite is directed at the hind limb, however, then one suspects
that the dog is displaying a grab or kill bite, thus indicating that the
motivation is predatory. One could
conclude that the dog is acting interspecifically with sheep and probably will
never be reliable (with sheep) or retrainable, since the critical period for social
development has passed.
Grab bites, crush bites, and dissect bites are specific motor patterns
in dogs and are thought to be homologous with wolf predatory behaviors. Among dogs, these predatory behaviors differ
with age and breed. Neonates do not
display them, and the onsets vary between and within breeds. Pointers and retrievers have grab bite, but
it is a fault for them to have crush bite ("hard mouth"). Grab bite is necessary for cattle-heeling
breeds, and not having it is a fault.
Grab bite is a fault in sheep-herding breeds. Livestock-guarding breeds must not have grab, crush, or
dissecting bites. In fact, they should
not even have chase as a motor pattern.
Good livestock-guarding dogs will not chase a ball, a behavior one
should try to elicit when judging a dog's fitness for protecting sheep. Good livestock-guarding dogs will not
(because they do not have the dissect motor pattern) open a stillborn calf to
feed. It is necessary to slice open
dead calves for a pen full of livestock-guarding dogs to feed, but not for
border collies.
CAN
MOTOR PATTERNS BE MODIFIED?
As stated, livestock-guarding dogs develop their social behavior
during the critical period from about 3 to 16 weeks of age. Evidently, the offset of this critical
period is delayed in some animals.
Emergence of predatory routines (if they appear) in livestock-guarding
dogs occurs after six months and occasionally after a year. Seldom do any of the predatory motor patterns
occur, although chase and grab bite are the most common. If, however, the dog has been properly
socialized with sheep, the later-emerging motor patterns do not get directed
toward sheep but may be directed to other species. This can lead to problems when, for example, dogs raised with
sheep are transferred to goats or when they disrupt wildlife on the sheep
range.
Border collies raised with sheep during the critical period of
socialization can be ruined as herding dogs.
Although such dogs might eye sheep, they seem unable to follow the eye
with stalk. In a double-blind
experiment in which the trainer did not know the background of the dogs, he
reported that the collie raised with sheep "couldn't hold the eye."
An individual may be capable of a motor pattern that it never displays
simply because it never gets the environmental signal that elicits it. Many puppies never use the lost call, for
example, because they are never lost.
Because of their early social experience, many livestock-guarding dogs
never display grab bite.
Some motor patterns must be temporally reinforced to become
functional. In many mammalian species,
maternal behaviors must be reinforced within a few minutes of parturition or
the dam will abandon the offspring.
Similarly, sucking by neonates must be reinforced within a critical
period or it is lost from the repertoire.
Animals born with mouth infections or correctable abnormalities often
lose the nursing motor pattern in the few days it takes to recover.
Other motor patterns are fixed in behavioral sequences and cannot be
displayed until the preceding behavior is performed. Some species of cats cannot eat carrion because the motor
patterns for consumption are connected sequentially to the whole predatory
repertoire. In some situations,
livestock-guarding dogs might be effective simply because they disrupt coyote predatory
routines by placing the coyote in conflicting motivational states that do not
allow it to complete the
sequence. Border collies have strong connections
between eye, stalk, and chase but weaker and modifiable connections with the
grab bite and kill bite. Trainers can
get a border collie to switch back and forth from chase to eye; but when it is
allowed to proceed from chase to grab bite, it becomes more difficult to
control the dog. Trying to get a wolf
not to go from chase to grab bite is even more difficult.
NEW BEHAVIORS FROM OLD MOTOR PATTERNS
In the evolution of behavior, motor patterns can become detached from
their ancestral sequence and reincorporated into a new sequence. Studies of dog breed differences give
insights into how these evolutionary feats are accomplished. The reader must remember that breeds are
allelically different and that allelic differences are differences in the
onset, rate, and offset of gene products.
Similarly, behavior should be described in terms of onset, frequency,
and offset of motor patterns.
As mentioned, the care-soliciting behaviors occur for a few weeks after
birth, whereas onsets of care-giving behaviors occur immediately after
parturition and are displayed for the next few weeks. The frequency of display is a response to several internal and
external variables.
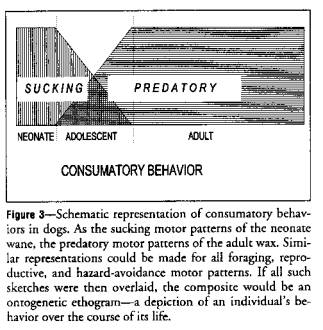
Sucking (feeding behavior) is an innate behavior that
appears shortly after birth (Figure 3).
It is expressed for long periods throughout the day for the first few
days, declines in frequency over the next few weeks, and disappears at about 8
weeks, never to reappear in the behavioral repertoire. Consumatory bites (feeding behavior) appear
in the repertoire at 4 weeks of age and increase in frequency until an adult
plateau is reached. They are maintained
for the rest of ontogeny.
Figure 3 shows that sucking does not grow or mature into feeding. Sucking offsets at about 8 weeks. Feeding onsets at about 4 weeks. Hall and Williams21 showed that
sucking behavior has little to do with feeding in either anatomical or
neurologic control. The display of
sucking motor patterns is initiated in one section of the brain and that of
consumatory bite motor patterns in another.
They are under separate motor control.
Each has separate motivations for performance. Satiation, for example, suppresses feeding
but not sucking.
One way to change innate behaviors is to shift onset and offset times
genetically. As one might expect, this
is one of the differences between breeds.
Border collies have early onsets of eye-stalk behaviors, whereas
livestock-guarding dogs have late or no onset of these behaviors.
Another way to change behavior genetically is to disconnect motor
patterns from other motor patterns.
Some species of wild cats have grab bite, kill bite, dissect, and
consume bites sequentially connected in the adult but not in the
adolescent. These different adult motor
patterns have separate onsets during adolescence and cannot be connected until
they are all in place. The timing of
the onset of these motor patterns may be due to developmental constraints or
they maj be the result of selection.
These interconnected feeding motor patterns may be the most efficient
way for the adult cat to feed; but at the same time, the connectedness
prevents them from eating carrion.
If young cats that cannot yet kill their own food had feeding motor
patterns sequentially connected, this sequencing would prevent them from eating
dead prey provided by the parent. In
this case, the adolescent may not be as efficient in killing prey but is more
adaptable than the adult because feeding behavior is not rigidly innate.
THE
ADAPTABLE ADOLESCENT
Neonatal and many adult species-typical behaviors have been discussed
as innately rigid and reflexive. The
adolescent, however, is quite a different story. Adolescence is so different that in many ways ethologists and
psychologists have difficulty understanding exactly what effect it has on adult
behavior. Nevertheless, this stage of
ontogeny deserves attention because it is the most difficult stage of development
to describe, it is the stage in which most mammals play and learn, and it is
the stage in which dogs, as a species, are trapped.
The most simplistic definition of adolescence is the period of
ontogeny between the neonate and the adult.
It is the period when the organism is experiencing the offsets of
neonatal motor patterns and concurrently the onsets of adult motor
patterns. Juvenile mammalian behavior
is a complex assemblage of waning neonatal and waxing adult motor patterns– the
period of metamorphic remodeling.22
In the neonatal stage, behaviors are preprogrammed (hard-wired). Neonatal feeding behaviors are all
accompanied by specialized anatomy that allows sucking to be performed. The neonate is not primitive or
underdeveloped. It is a highly
specialized organism in the sense that it is so perfectly adapted to its
environment it does not have to learn anything. There is no selective advantage to learning, nor is there enough
time or energy in the neonatal system to be able to experiment (learn)
behaviorally.
The adult mammal is also well adapted to its environment. Feeding, reproduction, and hazard-avoidance
behaviors have large innate components and often vary little or not at all
within a species. In general, most
adult mammals have difficulty rearranging sequences of motor patterns within a
specific behavioral repertoire; it is therefore difficult to modify their
behavior. They cannot afford the time
or energy to experiment.
Adolescence is a period of metamorphosis– anatomical remodeling. The neonatal organism is taken apart and
reconstructed into an adult. Behaviorally,
the individual is remodeled from innate neonatal feeding and hazard-avoidance
behaviors to the adult feeding, hazard-avoidance, and reproduction
systems. Sucking feeding behaviors do
not grow, or develop, into predatory feeding behaviors any more than the 18
feet of a caterpillar grow into the six legs of a butterfly. Instead, the animal is de-differentiated,
to borrow the words of the embryologist.
New organs are created de novo while old ones are discarded, just as the
highly complex placenta and its associated behaviors are discarded at
birth. Skulls do not grow from the
neonatal skull (the sucking skull) into an adult predatory skull. The neonatal skull is resorbed while the
adult skull is being laid down.23
Adolescents therefore have a changing access to neonatal and adult
motor patterns. The sequencing of
combinations of neonatal and adult motor patterns becomes the basis for play
and learning. Sequences of motor
patterns can be adjusted to the environment as the ever-changing adolescent
uses them in contextually new and varied ways.
As pieces of neonatal motor patterns are offset, the remainder are
displayed against the fragments of adult motor patterns. When pieces of neonatal motor patterns are
displayed in sequence with adult fragments in nonfunctional behavior patterns,
the behavior is called play. Putting
fragmented neonatal motor patterns together with adult behaviors in functional
ways is one definition of learning.
The distinction between learning and play is simply whether the resulting
motor sequence is functional and/or repeatable.
The two classes of vertebrates that play are mammals and birds, both
of which have well-defined neonates.
Play and learning are therefore considered artifacts of being able to
use motor patterns that are not contextually appropriate to their phylogenetic
origin. The hypothesis is that
adolescents play and learn so well because their neonatal behaviors have become
disconnected and their adult behaviors have not been sequentially aligned. Thus, they can use the dissociated motor
pattern of two distinct ontogenetic stages in new ways.
THE
PERMANENT ADOLESCENT
Dogs may be thought of as permanently adolescent. Genetically and evolutionarily they are
"arrested" in the adolescent period. They have become reproductive as adolescents. Retaining juvenile characteristics into the
adult period is called neoteny or paedomorphism by
embryologists. Dechambre24
suggested that canine breeds are differentially neotenic; whereas Fox,7 Frank
and Frank,25 and we26 suggested that not only are dogs
behaviorally neotenic, but breeds of dogs are differentially neotenic
behaviorally. The difference between
the two types of sheepdogs might simply be that livestock-guarding dogs are
arrested in a stage of development before the onset of adult predatory motor
patterns (e.g., grab, kill, and dissecting bites), whereas border collies are
in a stage that includes eye-stalk and chase but the grab and kill bites are
weak and imperfect and not sequentially linked to the rest of the repertoire.14
It is common practice to try to understand dog behavior by searching
for motor pattern homologues with wolves.
But wolf ontogeny should be taken into account. People often say that dogs are territorial
because wolves are and that wolves form packs with a leader, therefore dogs
form packs and the human trainer has to become the pack leader. Wolf cubs, however, do not have
reproductive territories or form packs and are not organized socially around a
leader. If dogs are neotenic, then they
should show behavior that is homologous to that of wolf cubs, not that
of wolf adults (e.g., territory defense or pack formation). Nor should a person pretending to be the
pack leader or dominant dog make any difference in their behavior. Wolf neonates and young adolescents do not
hunt, nor do the neonates have any predatory motor pattern. Wolf adolescents have some of the predatory
motor patterns, but they may not have them all, nor are the patterns connected
sequentially, depending on the individual's age.
Exploration of the behavioral differences in breeds does support the
hypothesis that dogs are arrested in an adolescent stage of wolf ontogeny. The difference between guarding and herding
sheepdogs is illustrative. The
guarding breeds never get to the ontogenetic stage when onsets of predatory
behavior occur. The herding breeds get
some of the predatory motor patterns, but not the final, wolf-like ones. Border collies and livestock guardians
raised from puppyhood together in one large pen separated themselves into distinct
"tribes" during play routines.
The border collies played eye-stalk-chase games, whereas the guardians
played at dominance-submission. The two
types of sheepdogs segregated, like species, and did not play with each
other. They are different
temperamentally because of the kinds and frequencies of motor patterns they
display.
The selection of type and intensity of play behaviors has differed
among breeds. For example, the performance
of sled dogs is based on selection for social play. What the driver thinks regarding his or her role as "pack
leader" is irrelevant to putting together a team. The driver can train them and discipline
them, but any attempt to become "dominant dog" is usually disruptive
to the racing potential of the team.
Many women with a gentle and supportive approach to training dogs excel
with their racing teams. Many teams
have female lead dogs. Many teams have
two lead dogs, which if one were "dominant" over the other would not
work. Many teams have several lead dogs
that race back in the team and can be swapped for a tired leader. None of this would be possible with a team
that behaved like adult wolves.
One should, however, treat the neoteny hypothesis with some
caution. It would be silly to think
that the ancestral wolf went through a maremma stage of behavior, then a
border collie stage, then a husky stage, to become finally an adult wolf. It is just as silly to think of a maremma as
representing a particular stage of wolf development, for the following reasons.
Puppies change (remodel) from neonates to adults. Heads grow (remodel) from little short-faced
puppy heads to big long-faced adult heads.
Change is allometric, not isometric.
To arrest an animal at a particular growth stage is in effect to
continue the allometric changes that are occurring at that stage. Since allometric changes are not prolonged
in the ancestral stage, the resulting dogs are anatomically bizarre. Indeed, dogs are anatomically bizarre
(e.g., head shapes or size variation).
If their
anatomy is novel in terms of their ancestor, then their behaviors will also be
novel and not directly equivalent to that of their ancestor. For this reason, one discussion of the
evolution of working dogs was ready to drop the notion of behavioral neoteny,
simply because although it might account for the heterochronic processes
resulting in dogs, it was a poor predictor of either anatomical or behavioral
result.27 In the five years
that have elapsed since the paper was written, optimism about the use of
neoteny as a predictor of anatomy and behavior is returning.
CONCLUSION
The foregoing discussion of the selection process for behavioral
differences among canine breeds has focused on breeds that were developed for
work. This discussion underscores the
biological bases of behavior, emphasizing details of physical structures that
enable or limit behavior. We believe,
however, that this information will also provide new ways of looking at the
behavior of companion animals and offer alternative interpretations of problem
behaviors. Such increased understanding
of behavior should lead to improved techniques for the prevention, treatment,
and management of canine behavior problems.